Following the call for applications for Rare diseases (3rd Public Health Programme) in support of the setup of new registries, the Brains for Brain Foundation is proud to announce that the MetabERN proposal for the creation of the Unified Registry for Inherited Metabolic Disorders (U-IMD) has been approved by the EU.
The major objective of this project 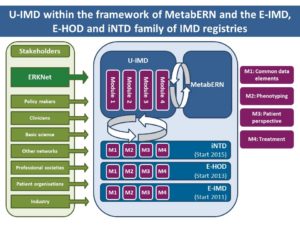 s to establish the first unified European registry that encompasses all inherited metabolic disorders (IMDs) as listed by Orphanet (http://www.orpha.net/). The overall aim of this project is to promote health for children, adolescents and adults affected by rare IMDs and to reduce variation between countries and enable and empower patients, wherever they live, to access the necessary expertise and services and promote research on IMDs and the development of safe and efficacious new treatments. Furthermore, U-IMD will systematically collect data of affected individuals with an IMD of yet unidentified molecular origin and will group them according to their clinical and biochemical phenotype. This will help to identify and systematically treat and follow these patients once the etiology of their disease has been clarified.
s to establish the first unified European registry that encompasses all inherited metabolic disorders (IMDs) as listed by Orphanet (http://www.orpha.net/). The overall aim of this project is to promote health for children, adolescents and adults affected by rare IMDs and to reduce variation between countries and enable and empower patients, wherever they live, to access the necessary expertise and services and promote research on IMDs and the development of safe and efficacious new treatments. Furthermore, U-IMD will systematically collect data of affected individuals with an IMD of yet unidentified molecular origin and will group them according to their clinical and biochemical phenotype. This will help to identify and systematically treat and follow these patients once the etiology of their disease has been clarified.
The Unified European Registry for Inherited Metabolic Disorders (U-IMD) will be established in collaboration with the MetabERN.
As laid down in Article 12 of the Directive on the application of patients’ rights in cross-border healthcare, registries constitute one of the important objectives of the European Reference Networks (ERNs) to be set up and consequently this achievement is of great value for the MetabERN and represents a major milestone for the entire network of ERNs.
The U-IMD Registry takes its origin from 3 existing and well characterised registries: (E-IMD: https://www.eimd-registry.org/, E-HOD: https://www.ehod-registry.org/, iNTD: https://www.intd-registry.org/) family of IMD registries, and will have a relevant impact on improving the health of patients with Inherited Metabolic Disorders and facilitates post- authorisation safety studies (PASS) for orphan drugs. In particular it will enable systematic deep clinical phenotyping, genotype/phenotype correlation, interdisciplinary analysis of the disease courses, diagnostic approaches, current treatment strategies and quality of life of IMD patients on a European level. Beside the extension of knowledge, U-IMD will also contribute to better treatment strategies, resulting in improved long-term prognosis of patients.
The project consists of 3 components: (1) a novel registry platform for all known IMDs, (2) an upgrade of existing IMD registries and (3) a collaboration with the European Rare Kidney Disease Reference Network (ERKNet). The new Unified European Registry for Inherited Metabolic Diseases (U-IMD) will encompass all known IMDs, fully implementing EUCERD recommendations. The data modules developed for U-IMD will be integrated in the existing IMD registries, with the iNTD registry as pilot, thus reaching interoperability of patient records. MetabERN will develop a common standard for minimal core data sets also with the ERKNET, the ERN for Kidney Diseases, to show interoperability and possible expansion of the registry to all other rare diseases.
The U-IMD registry will follow an open multiple stakeholder approach, explicitly seeking collaborations with national and EU level health authorities, other scientific networks and consortia, patient and parent organizations and industry.
The Project is lead by Stefan Kölkler ( MetabERN Vice-coordinator, University of Heidelberg, DE) and it is at the moment at the Grant Agreement status.
The network that has been formed for the proposal comprises the following MetabERN Healthcare Providers: the University of Heidelberg, the Hospital Sant Joan de Deu (ES), the Bambino Gesú Hospital (IT), the University of Prague (CZ), The Horst Schmidt Kliniken (DE).






 Prof Scarpa main key tasks are the following:
Prof Scarpa main key tasks are the following: 24 European Reference Networks (ERNs) were formally launched on 9 March 2017. Representatives from the networks and Member States, along with patients and policymakers, gathered in Vilnius, Lithuania, for the 3rd ERN Conference and kick-off meetings (9 & 10 March).
24 European Reference Networks (ERNs) were formally launched on 9 March 2017. Representatives from the networks and Member States, along with patients and policymakers, gathered in Vilnius, Lithuania, for the 3rd ERN Conference and kick-off meetings (9 & 10 March).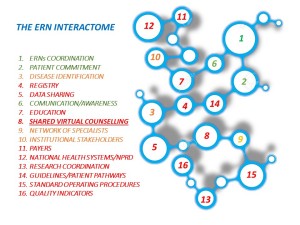 ERNs. “There is crosslinking in nature,” said Dr Scarpa. “Nothing is isolated.” He said ERNs should be at the frontier of ‘network medicine’ – a new way of thinking of health which breaks down silos between disciplines and disease taxonomies. Tools must be developed to link the ERNs, including a shared virtual counselling system that allowed members of different ERNs to discuss complex cases. “ERNs are not a project or a programme they are a concept – a revolution in the understanding of rare diseases,” he said. “Don’t think of ERNs as discrete networks but as a large task force for sharing expertise.”
ERNs. “There is crosslinking in nature,” said Dr Scarpa. “Nothing is isolated.” He said ERNs should be at the frontier of ‘network medicine’ – a new way of thinking of health which breaks down silos between disciplines and disease taxonomies. Tools must be developed to link the ERNs, including a shared virtual counselling system that allowed members of different ERNs to discuss complex cases. “ERNs are not a project or a programme they are a concept – a revolution in the understanding of rare diseases,” he said. “Don’t think of ERNs as discrete networks but as a large task force for sharing expertise.”


