The members of the “conect4children” (c4c) initiative today announced the start of a large
collaborative paediatric network that will facilitate the development of new drugs and other
therapies for the entire paediatric population in Europe.
The conect4children (collaborative network for European clinical trials for children, (c4c) consortium aims to enhance the competitiveness of Europe as a critical region for developing medicines for children by using existing expertise, patient access and developing common processes to be applied to disease natural history studies, registries, studies of new therapies and comparisons of existing therapies.
The consortium is a novel collaboration between academic and private sectors that includes 33 academic and 10 industry partners from 20 European countries, more than 50 third parties and around 500 affiliated partners.
The six-year project, comprised of a multidisciplinary public-private consortium, brings together key stakeholders across academia and industry. It is a pioneering opportunity to build capacity for the management of multinational paediatric clinical trials across Europe whilst ensuring the voices of children, young people and their families are heard. Strong links with regulators will be established.
There are many scientific and operational challenges faced by both pharmaceutical companies and academia when running paediatric clinical trials. According to Prof. Carlo Giaquinto of Fondazione PENTA Onlus and University of Padova, who coordinates the project, “c4c will address critical problems with the design, implementation and
operational conduct of paediatric clinical trials, such as fragmented and redundant efforts between sponsors, sites and countries; the paucity of patients available for study in many paediatric indications and the need for multiple capable sites and expertise to make trials successful.”
This project aims to generate a sustainable infrastructure that optimises the delivery of clinical trials in children through:
• a single point of contact for all sponsors, sites and investigators
• efficient implementation of trials adopting consistent approaches, aligned quality standards and coordination of sites at national and international level
• collaboration with specialist and national networks
• high quality input to study design and preparation through rigorous strategic and operational feasibility assessment
• the promotion of innovative trial design and quantitative science methods
• an education and training platform to shape the future leaders of paediatric drug development
• the development of sustainable support for all these activities
One of the key goals of the project is to support the use of innovative trial designs and new quantitative methods to foster development of new innovative medicines and to support development in rare paediatric diseases and high medical need area “Children must have access to innovative medical therapies that have been developed with the same degree of urgency and rigor as those for adults,” said Joanne Waldstreicher, MD, Chief Medical Officer, Johnson & Johnson. “With conect4children in Europe joining in this effort, complementing work under way with I-ACT for Children in the United States, we will be able to accelerate the availability of high quality scientific data
that can improve the safe and effective use of therapies in children.”
“Clinical trials with medicinal products for paediatric use are one of the most sensitive areas in science – both from a medical and an ethical perspective”, said Dr. Michael Devoy, Chief Medical Officer of Bayer. “Improving the clinical trial infrastructure is an import step in enabling children to take part in medical progress”.
Dr. Mark Turner, Co-coordinator of the project, University of Liverpool, stated: “This network will have a significant impact on how we develop much-needed innovative and improved medicines for babies, children and young people. A number of collaborations built up over the past decade will contribute to this pan-European research network. The
University of Liverpool is proud to be collaborating with institutions and research networks across Europe”
With a budget of about 140 Mio€ (IMI2 support of 67 Mio€ and industry partners’ in-kind contribution of 73 Mio€), c4c is one of the biggest initiatives funded by the Innovative Medicines Initiative 2 Joint Undertaking (IMI2 JU) under grant agreement n º 777389. The Innovative Medicines Initiative 2 Joint Undertaking is Europe’s biggest Public Private Partnership and is funded jointly by the European Union´s Horizon 2020 research and
innovation programme and the European pharmaceutical industry (represented by EFPIA, the European Federation of Pharmaceutical Industries and Associations).
• More info on IMI: www.imi.europa.eu
• Follow IMI_JU on Twitter: @IMI_JU
The parties involved
The project is coordinated and led by: Fondazione PENTA – for the treatment and care of children with HIV – ONLUS, The University of Liverpool, Janssen Pharmaceutica NV, Bayer AG Other partners are: Ospedale Pediatrico Bambino Gesù; EURORDIS – European Organisation for Rare Diseases Association; European Cystic Fibrosis Society; Stichting Katholieke Universiteit; Swiss Clinical Trial Organisation Verein; Associação para Investigação e Desenvolvimento da Faculdade de Medicina; Istituto Giannina Gaslini; University College London; SIOP Europe ASBL; Tartu Ulikool; Okids GMBH; University of Newcastle upon Tyne; Universiteit Gent; Universitaetsklinikum Heidelberg; Aristotelio Panepistimio Thessalonikis; Instytut Pomnik Centrum Zdrowia Dziecka; Helse Bergen
HF*Haukeland University Hospital; ECNP Research & Scholarship Foundation; Robert Bosch Gesellschaft fur Medizinische Forschung MBH; University College Cork– National University of Ireland, Cork; Karolinska Institutet; Fundacio Sant Joan de Deu; Servizo Galego de Saude; Gyermekgyógyászati Klinikai Vizsgálói Hálózat; Fondazione per la Ricerca Farmacologica Gianni Benzi Onlus; ECRIN European Clinical Research Infrastructure Network; The Hospital District of Helsinki and Uusimaa; Institut National de la Sante et de la Recherche Medicale; HSK DR Horst Schmidt Kliniken Wiesbaden Gmbh; ARSENAL.IT-Centro Veneto Ricerca e Innovazione per la Sanità Digitale; Univerzita Karlova; Sanofi-Aventis Recherche & Développement; Eli Lilly and Company Limited; UCB Biopharma SPRL; Novartis Pharma AG; Institut de Recherches Internationales Servier; GlaxoSmithKline Research and Development LTD.; Pfizer Limited; F. Hoffmann – La Roche AG
The full list of organisations involved in the project can also be found at the c4c webpage
www.conect4children.org
Project Office/General Enquires: Email us. communication@conect4children.org
Disclaimer This communication reflects the views of the c4c Consortium and neither IMI nor the
European Union and EFPIA are liable for any use that may be made of the information
contained herein
H2020-JTI-IMI2-2016-10. Proposal: 777389






 French and Canadian scientists make discovery that could affect diagnosis, genetic counselling and therapeutic approaches in patients with a rare conditionMontreal – Rare hereditary recessive diseases were thought to be expressed in off-spring only when both parents carry a mutation in the causal gene, but a new study is changing this paradigm. An international research team led by scientists at the University of Lorraine in France along with McGill University and the Research Institute of the McGill University Health Centre (RI-MUHC) in Canada discovered a new cause of a rare condition known as cblC, that they named “epi-cblC”. They reported it in patients from Europe and the United States. Patients who have cblC are not able to process *vitamin B12, leading to severe health problems.cblC is usually caused by two mutations – one inherited from each parent – in a gene called MMACHC. In some patients, the scientists found this disease actually results from a mutation on a single copy of the gene and the silencing of the second copy by a gene modification referred to as epimutation. This epimutation is produced by a mutation in an adjacent gene. Their findings, which were published this month in Nature Communications, may have an impact on diagnosis, and genetic counselling in families with genetic diseases, as well as in the development of new therapeutic approaches.
French and Canadian scientists make discovery that could affect diagnosis, genetic counselling and therapeutic approaches in patients with a rare conditionMontreal – Rare hereditary recessive diseases were thought to be expressed in off-spring only when both parents carry a mutation in the causal gene, but a new study is changing this paradigm. An international research team led by scientists at the University of Lorraine in France along with McGill University and the Research Institute of the McGill University Health Centre (RI-MUHC) in Canada discovered a new cause of a rare condition known as cblC, that they named “epi-cblC”. They reported it in patients from Europe and the United States. Patients who have cblC are not able to process *vitamin B12, leading to severe health problems.cblC is usually caused by two mutations – one inherited from each parent – in a gene called MMACHC. In some patients, the scientists found this disease actually results from a mutation on a single copy of the gene and the silencing of the second copy by a gene modification referred to as epimutation. This epimutation is produced by a mutation in an adjacent gene. Their findings, which were published this month in Nature Communications, may have an impact on diagnosis, and genetic counselling in families with genetic diseases, as well as in the development of new therapeutic approaches.
 Christine has played an outstanding role in our Lysosomal storage diseases community and for the entire field of rare inherited diseases.
Christine has played an outstanding role in our Lysosomal storage diseases community and for the entire field of rare inherited diseases. Prof. Maurizio Scarpa was invited to actively participate to the meeting organised by DG Research and Innovation (DG RTD), European Commission, to represent the MetabERN and particularly contributing to the Gastein session “Personalising healthcare: How rare diseases pave the way” that took place on Wednesday 4 October 2017 from 09.00 to 11.00 am.
Prof. Maurizio Scarpa was invited to actively participate to the meeting organised by DG Research and Innovation (DG RTD), European Commission, to represent the MetabERN and particularly contributing to the Gastein session “Personalising healthcare: How rare diseases pave the way” that took place on Wednesday 4 October 2017 from 09.00 to 11.00 am.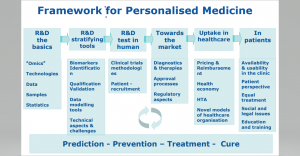 During the session the different stakeholders discuss about current and future way to exchange knowledge and develop strategies, policies and guidance that pave the way to personalised medicine in Europe, using rare diseases as a model. Advances in genomics and other “omics” technologies have significantly improved our understanding of the pathogenesis of rare diseases. This has opened avenues for piloting new, personalised diagnostic methods and therapies. The impact of “omics” is reinforced by the combination of these data with Real-World Data (RWD).
During the session the different stakeholders discuss about current and future way to exchange knowledge and develop strategies, policies and guidance that pave the way to personalised medicine in Europe, using rare diseases as a model. Advances in genomics and other “omics” technologies have significantly improved our understanding of the pathogenesis of rare diseases. This has opened avenues for piloting new, personalised diagnostic methods and therapies. The impact of “omics” is reinforced by the combination of these data with Real-World Data (RWD). This meeting was aimed to create collaboration among the Italian HCPs involved in the European Reference Networks (ERN), that as highlighted by Andrzej Jan Rys (Director HelathSystems, Medical Products and Innovation, DG Sante’) are virtual networks of specialists officially born in March 2017, dedicated to the diagnosis and treatment of diseases complex or rare. The 24 ERNs so far approved cover all major disease groups and represent almost a thousand of multidisciplinary medical teams of more than 300 hospitals, located in 25 EU Member States and Norway.
This meeting was aimed to create collaboration among the Italian HCPs involved in the European Reference Networks (ERN), that as highlighted by Andrzej Jan Rys (Director HelathSystems, Medical Products and Innovation, DG Sante’) are virtual networks of specialists officially born in March 2017, dedicated to the diagnosis and treatment of diseases complex or rare. The 24 ERNs so far approved cover all major disease groups and represent almost a thousand of multidisciplinary medical teams of more than 300 hospitals, located in 25 EU Member States and Norway.

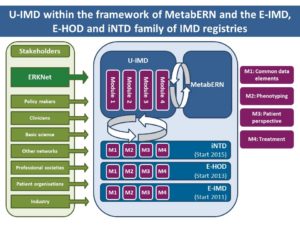 s to establish the first unified European registry that encompasses all inherited metabolic disorders (IMDs) as listed by Orphanet (http://www.orpha.net/). The overall aim of this project is to promote health for children, adolescents and adults affected by rare IMDs and to reduce variation between countries and enable and empower patients, wherever they live, to access the necessary expertise and services and promote research on IMDs and the development of safe and efficacious new treatments. Furthermore, U-IMD will systematically collect data of affected individuals with an IMD of yet unidentified molecular origin and will group them according to their clinical and biochemical phenotype. This will help to identify and systematically treat and follow these patients once the etiology of their disease has been clarified.
s to establish the first unified European registry that encompasses all inherited metabolic disorders (IMDs) as listed by Orphanet (http://www.orpha.net/). The overall aim of this project is to promote health for children, adolescents and adults affected by rare IMDs and to reduce variation between countries and enable and empower patients, wherever they live, to access the necessary expertise and services and promote research on IMDs and the development of safe and efficacious new treatments. Furthermore, U-IMD will systematically collect data of affected individuals with an IMD of yet unidentified molecular origin and will group them according to their clinical and biochemical phenotype. This will help to identify and systematically treat and follow these patients once the etiology of their disease has been clarified.